A new RNA vaccine will soon join the global fight against COVID-19. CureVac, a German pharmaceutical company, has been working on a vaccine against the coronavirus and is expected to announce the results of its late-stage clinical trials next week. If it’s found to be safe and effective, it will be the third RNA vaccine, after Pfizer BioNTech and Moderna. And it could be a gamechanger.
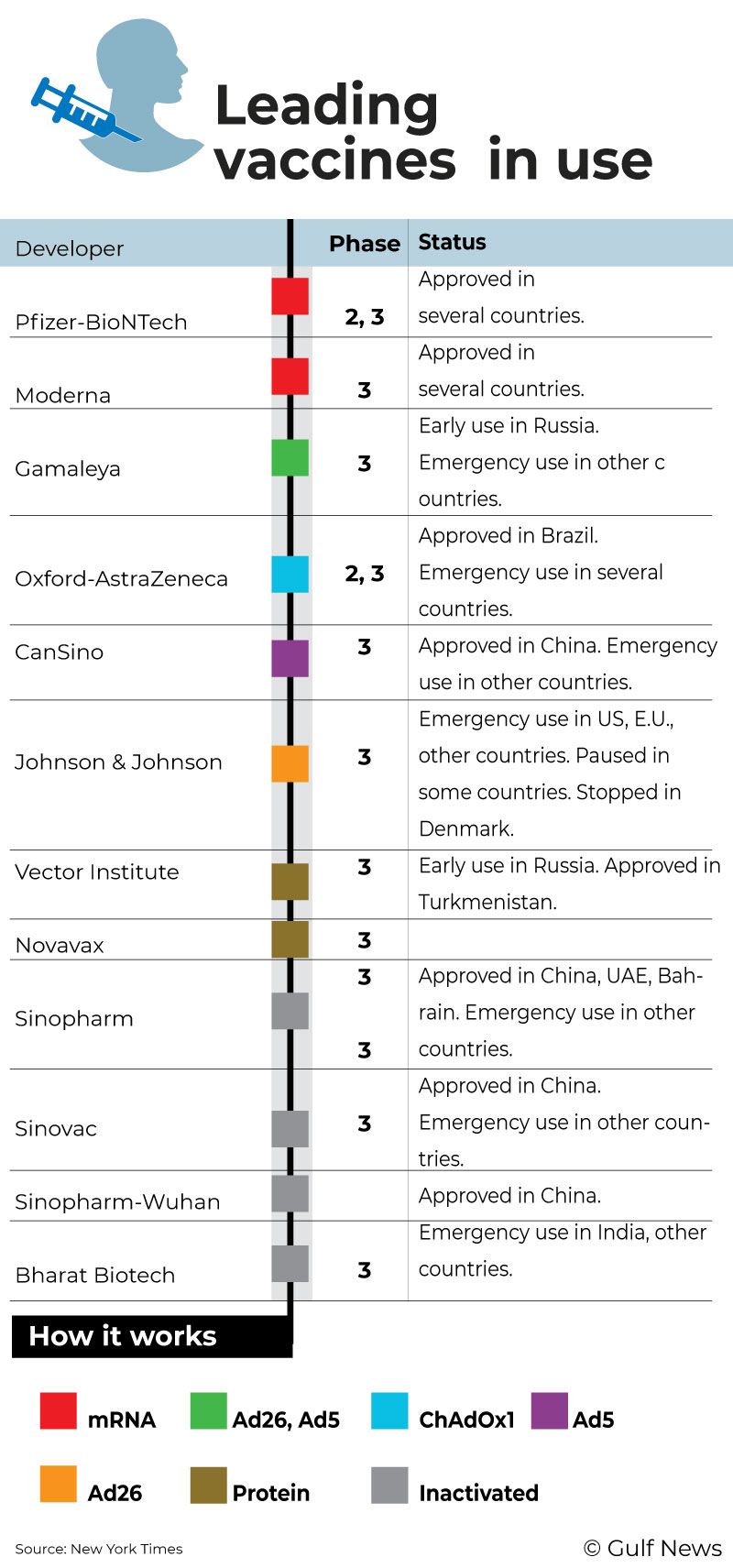
Pfizer and Moderna vaccines have been successful in reining in coronavirus infection, having provided protection to millions of people in more than 90 countries. The two are pathbreakers in vaccine technology. They use the mRNA (messenger RNA) route to implant genetic information in the human body to produce the coronavirus spike protein, which will provoke a response from the immune system.
All RNA (ribonucleic acid) vaccines are generally required to be kept in a deep freeze. That poses problems in transportation and storage. Pfizer had circumvented it by making custom-made boxes to maintain freezing temperature during transit and in warehouses.
Here’s where CureVac scores. It doesn’t have to be kept in a deep freeze. Ordinary refrigerators would do, making the vaccines accessible in many parts of the world, especially places hardest hit by the SARS-CoV-2 virus.
What’s CureVac’s COVID-19 vaccine candidate?
CVnCoV is a vaccine candidate made by CureVac that uses unmodified, natural mRNA to trigger the production of the spike protein of SARS-CoV-2. Its targeted optimisation can induce high levels of protein protection in the cells with low mRNA doses, according to the company website.
After the vaccine is injected into the body, it prompts the production of spike proteins. That activates the immune cells to produce antibodies and T-cells to fight it.
What’s its dosage?
The vaccine is delivered as a muscle injection of two doses administered four weeks apart.
How’s CureVac different from other RNA vaccines?
All RNA vaccines have to be kept in a deep freeze because they are fragile and degrade quickly. That has been a stumbling block in their storage and distribution. So RNA vaccines haven’t reached much of the developing world, which do not have an uninterrupted power supply. And the lack of funds rules out the purchase of deep freezers.
Here’s where CureVac will come in handy if the trial results are encouraging. If it is found to be safe and effective and obtains approvals from health regulators, CureVac could help immensely in the fight against COVID-19. Since it can be stored in standard refrigerators at 36–46°F (2–8°C), the CureVac vaccine can be stored and transported more easily than the other RNA vaccines.
How does the CureVac jab remain stable?
CureVac’s researchers found a method to put the RNA molecules in fatty bubbles to prevent them from disintegrating, enabling the vaccine to remain stable at relatively warm temperatures. So it doesn’t need a deep freezer; a standard refrigerator would suffice. The vaccine can be kept in a room (outside the fridge) for 24 hours before injecting it.
According to the US Centers for Disease Control and Prevention, encapsulating mRNA in a protective shell will prevent its degradation and improve vaccine effectiveness.
more on COVID-19 VACCINES
How soon will it be available?
CureVac launched a Phase 3 trial in December 2020, with 36,500 volunteers in Germany. In February, the European Union began a rolling review to speed up approval if the Phase 3 trial results are promising. The results are expected next week, and if they are positive, CureVac can submit requests for approval. Since the approvals are dependent on the health regulators of each country, it’s difficult to put a time frame.
Who are CureVac’s partners?
CureVac has been a laggard among RNA vaccine developers partly due to funding issues. Germany’s BioNTech benefited from their partnership with US pharmaceutical giant Pfizer, while Moderna allied with the National Institutes of Health and received a billion dollars from the US government’s Operation Warp Speed. So they were able to speed up vaccine development.
The Coalition for Epidemic Preparedness Innovations, a foundation that supports vaccine research, gave $34 million for CureVac’s research in 2019. In June 2020, the German government invested around $360 million. Other support came from the Bill & Melinda Gates Foundation and billionaire Dietmar Hopp, the co-founder of German software giant SAP.
Last year, German newspapers reported that US President Donald Trump had offered CureVac $1 billion to move its operations to America. But the company denied the reports.
Has CureVac lost the vaccine race?
Pfizer and Moderna have snapped up vaccines deals from much of the developed world. But CureVac’s better stability at relatively warmer temperatures will help secure the agreements to provide vaccines to underdeveloped countries. Besides that, they already have contracts with the EU, Tesla and several pharma giants.
Bayer, Celonic, GSK, and Novartis have thrown in their support for the production of the CureVac vaccine and for developing new ones to combat newer strains of the coronavirus. These companies will support the production of 150 million doses in 2021 and up to 360 million doses in 2022.
CureVac has a deal to provide the EU with 225 million doses of their vaccine. It has also linked up with Elon Musk’s Tesla to create mRNA “micro-factories” that could be set up worldwide to make the vaccine.
What are the production and supply targets of CureVac?
CureVac says it can manufacture up to 300 million doses this year and ramp it to a billion doses next year. With Tesla’s help, the mRNA “micro-factories” could make billions of doses of the vaccine.
The German firm says US export restrictions on key materials are affecting its short-term supply in Europe. “Due to the Defence Production Act, we are not getting certain goods out of the USA,” CureVac Chief Executive Franz-Werner Haas told the German weekly Der Spiegel. “We are not getting all the materials that we need,” he said.
The Act gives US federal agencies the power to decide on procurement orders related to national defence, but it has also been used in times of natural disasters.
That would slow CureVac’s vaccine production. The world can’t have enough vaccines. Every additional dose would help.
from World,Europe,Asia,India,Pakistan,Philipines,Oceania,Americas,Africa Feed https://ift.tt/33latNJ
No comments:
Post a Comment