Can we mix vaccines? The question is significant now since COVID-19 vaccination campaigns worldwide are underway, and the production and distribution of jabs have not kept pace. Two different vaccines for the first and second doses have been seen as the solution to swiftly inoculate populations to achieve the desired herd immunity that slows down the infection rate.
The two-vaccine strategy is fraught with risks as it is a relatively new frontier, despite attempts to fight HIV (the virus that causes AIDS) and Ebola with multiple vaccines. There have been some studies on the efficacy of using two drastically different vaccines against COVID, but the data is not adequate to advocate it as a strategy. That hasn’t stopped many countries from pursuing it.
Is it dangerous to combine two vaccines?
The World Health Organisation has warned against using different COVID-19 vaccines for the first and second doses. That chimes in with the UAE advice against using two vaccines.
In contrast, several countries in Europe and elsewhere have approved the combining of vaccines from two different platforms. But experts say more studies are required to ensure the safety of the two-vaccine strategy.
“It’s a little bit of a dangerous trend here,” Soumya Swaminathan, WHO chief scientist, told an online briefing on Monday. “It will be a chaotic situation in countries if citizens start deciding when and who will be taking a second, a third and a fourth dose.”
Why mix vaccines?
A multiple-vaccine strategy involving doses from different platforms is called heterologous prime-boost vaccination. Since two vaccines boost the immune system in different ways, multiple vaccines are expected to provide broader coverage.
Heterologous prime-boost was started in the 1990s by HIV researchers since a classical vaccine would not induce the extremely complex immunological mechanisms needed for potential protection from HIV infection, according to Dr Pierre Meulien, executive director of the Innovative Medicines Initiative (IMI), an EU and European pharmaceutical industry partnership.
The first clinical heterologous vaccine was an Ebola vaccine approved in May 2020, while the Sputnik V COVID vaccine is also heterologous, reports say.
“Mixing vaccine platforms has a long history in immunology as being far superior to multiple doses of the same vaccine,” says Ross Kedl, an immunologist at the University of Colorado Anschutz School of Medicine.
For COVID-19, a widespread policy of mixing vaccines could also help to ease vaccine production and supply bottlenecks. It can only be employed as a vaccination strategy only if there are more data to reaffirm the positive results from the preliminary tests.
What the studies on vaccine combinations say?
There’s been only a handful of studies on the efficacy of mixing different COVID vaccines, and that’s the worry. Right now, this is a “data-free area”, according to Soumya Swaminathan. “Data from mix and match studies of different vaccines are awaited — immunogenicity and safety both need to be evaluated, she added.”
At least four studies looked at the feasibility of mixing AstraZeneca and Pfizer-BioNTech vaccines in separate doses. The Spanish research Combivac found that the combination was highly effective in producing a robust immune response from the body; the results are published in the journal Nature. Trials by researchers in Saarland University and Ulm, Germany, also supported these findings, although they have not been fully evaluated.
According to the amount of data we have, the best option is still not to mix vaccines.
The results from the University of Oxford’s Com-COV study showed that the side-effects were mild and moderate reactions. According to the study published in the journal Lancet, participants reported increases in chills, fatigue, headache, joint pain, malaise, and muscle ache. Fatigue and headache were more frequent when the vaccines were mixed compared to two rounds of the same jab.
Matthew Snape, associate professor in Paediatrics and Vaccinology at the University of Oxford and chief investigator on the study, said the results are promising but cautions “they don’t resolve whether any improvement in T cell response results from longer dose intervals rather than the mixing”.
“It is important that we inform people about these data, especially as these mixed-doses schedules are being considered in several countries,” said Snape said. “Importantly, there are no safety concerns or signals, and this does not tell us if the immune response will be affected,” he added.
“So far, according to the amount of data we have, the best option is still not to mix vaccines,” Bloomberg quoted Ramon Lorenzo Redondo, a molecular virologist at Northwestern University, Illinois, US, as saying.
What happens when you mix vaccines?
“Mixing two types of vaccines may give the immune system multiple ways to recognise a pathogen. The mRNA vaccines are excellent at inducing antibody responses, and the vector-based vaccines are better at triggering T-cell responses giving a strong immune response,” Dr Ramesh Bhaskaran, Internal Medicine (specialist), Aster Hospital, Ghusais, Dubai.
But mixing vaccines may carry the additional risk of serious adverse effects too, the Dubai-based doctor cautioned.

Is it safe to mix vaccines?
“There is no exact data about the safety of mixing COVID vaccines. However, a small study published in the medical journal Lancet recently showed a slight increase in intensity and duration of the minor side effects like pain, fever and fatigue. No major adverse event was reported on mixing vaccines,” said Dr Idrees Mubarik, Endocrinology (Specialist), Aster Hospital, Mankhool and Ghusais, Dubai.
What happens when a vaccine is administered?
“In simple words, we can say vaccines work by mimicry. For instance, the mRNA vaccine provides a template for our immune cells to make and identify viral proteins without being exposed to the virus. Immune cells recognise that protein and attack it whenever they find it again. That’s how they dampen the intensity of subsequent infection if that happens,” said Dr Idrees Mubarik.

COVID-19 vaccines and their platforms
“COVID-19 vaccines are being developed using several different platforms. The major antigenic target is the large surface spike protein. Immune responses to a SARS-CoV-2 inactivated vaccine (Sinopharm) would target not only the spike protein but also other components of the virus.
“Pfizer and Moderna vaccines employ messenger RNA, which induces our cells to produce viral proteins that stimulate a potent immune response. They contain material from the virus that causes COVID-19 that gives our cells instructions for making a harmless protein that is unique to the virus. After our cells make copies of the protein, they destroy the genetic material from the vaccine. Our bodies recognise these proteins and build T-lymphocytes and B-lymphocytes that will remember how to fight the virus if we are infected in the future.
The AstraZeneca vaccine uses a chimpanzee adenovirus to stimulate the immune system to produce antibodies. Basically, the vehicle for delivery of the spike protein is different, and it induces immunity by targeting different aspects of our immune system,” Dr Bhaskaran said.

What are the different vaccine types, and how do they work?
Vaccines prime people’s bodies to fight a pathogen (virus, bacteria or disease-causing molecule), thus preventing a severe infection, even if exposed. If individuals contract COVID-19, for example, now vaccines are available to protect them from getting seriously ill, eliminating the need for hospitalisation in most cases. And they will recover faster. That, at least, is the theory.
By “training” the body’s immune system, vaccines help develop resistance — by literally imitating an infection — with the sole aim to kick up the body’s natural immune response specific to that pathogen (such as SARS-CoV-2).
In general, there are six vaccine types:
- Live-attenuated/non-replicating vector vaccines
- Inactivated (killed virus)
- Sub-unit, recombinant, polysaccharide, conjugate vaccines
- Toxoid
- DNA
- messenger RNA (mRNA)
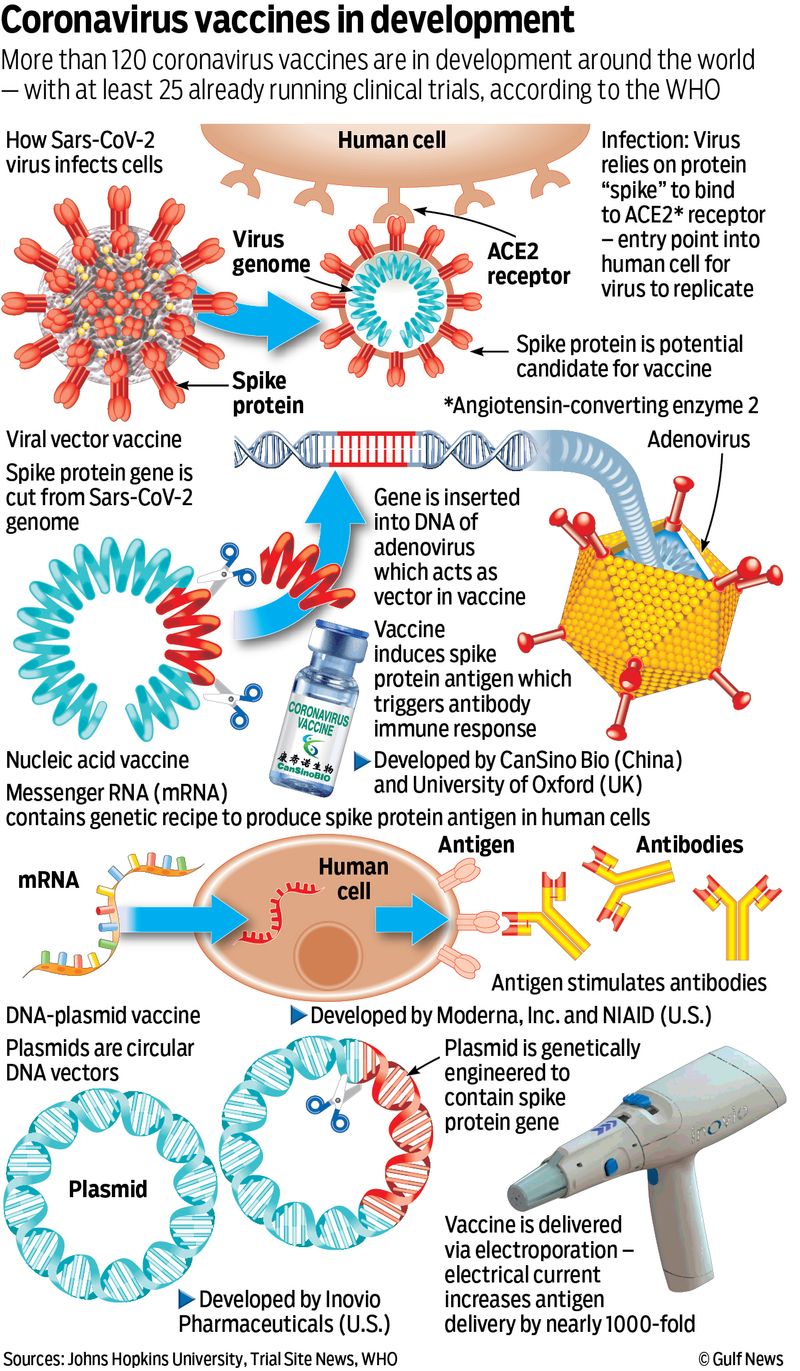
Among the 19 approved vaccines against SARS-CoV-2, there are at least 4 “platforms” (or types).
- Live-attenuated/non-replicating viral vector vaccines (i.e. AstraZeneca, Cansino, Covaxin, Covishield, Janssen/J&J, and Sputnik Light, Sputnik V): Viral vectors are tools commonly used by biochemists to deliver genetic material (the Spike protein of SARS-CoV-2) into cells. This process can be performed inside a living organism (like an Adenovirus) or in cell culture.
- Sub-unit vaccine (i.e. Dimer, EpoVacCorona): A sub-unit vaccine presents one or more antigens to the immune system — thus skipping the need to introduce pathogen particles, whole or otherwise. “Subunit” means the antigen is a fragment of the pathogen/. The antigens involved can be any molecule, such as proteins, peptides or polysaccharides.
- Inactivated/killed virus vaccine (i.e. Covaxin, Sinopharm, CoronaVac/Sinovac): The serum comes from the whole virus or a version of it. It is cultured in huge quantities and processed/mass-produced in a high-security biosafety facility. They are then “killed” before being stored in vials for distribution. In effect, the dose injected to us (determined after careful, extensive trials) gets us “infected”, which then triggers an immune response in our body. That’s how immunity is achieved with this platform.
- mRNA (i.e. Pfizer/BioNTech, Moderna/NIH, and Takeda’s TAK-919): This type of vaccine teaches your body how to make a protein that will trigger an immune response without using a live virus. After you get an mRNA vaccine, your body produces antibodies that help fight the infection if a virus enters your body in the future.
These are totally different methods of vaccine development and even manufacturing processes, so mixing-and-matching them needs extensive clinical trials to ensure safety and efficacy.
Have multiple vaccines been administered previously?
“In polio, different types of vaccines have been used, both oral and injectable vaccines have been used in some children with reassuring safety,” said Dr Mubarik.
Dr Bhaskaran said: “Immunologists have tested mixing doses of different vaccines for long. HIV researchers have long been exploring this for HIV vaccination. The Ebola vaccine is an example of an effective mixed-product vaccine.
The first shot uses the same adenovirus vector as AstraZeneca’s vaccine, and the second uses a modified version of a Poxvirus called Modified Vaccinia Virus Ankara (MVA). These are two different types of pneumococcal vaccines that have different mechanisms of action, and in certain situations, we recommend boosting one with the other.”
What doctors think of mixing COVID-19 vaccines
“My personal opinion regarding the mixing of vaccines is that it should not be routinely done until further safety data emerges. Also, it would have been much better if travel guidelines by different countries emphasise vaccination in general rather than any particular brand of vaccine,” said Dr Mubarik.
“The serious risks of rare side effects is one reason some researchers recommend that people stick to the standard two shots of a single vaccine for now. When combining two different vaccines, both of which might have their own profile of adverse events and effects, could amplify problems. When given an option of either getting a mixed schedule or no second dose, then you certainly go for the mixed schedule,” said Dr Bhaskaran.
READ MORE
Why do people mix vaccines?
When was the last time you asked a doctor which company manufactured the flu vaccine or polio jab you received? The chances are that you never did.
During the peak of the COVID-19 pandemic, most people hoped that a vaccine to keep the coronavirus at bay would be manufactured soon. When the vaccines arrived, people stood in lines to get their jab.
Then why is there a fuss about the vaccine today?
There are two primary reasons: travel and the search for a better vaccine.
Vaccines for travel
Do not travel internationally until you are fully vaccinated, is the general advice by the health authorities in most countries. Some have gone further and listed the vaccines needed to be taken to enter a particular country. This creates a problem for travellers, prompting some to mix and match vaccines to gain entry into specific countries.
Take, for example, the digital COVID-19 certificate introduced by the European Union. It allows EU residents to move freely in the 27-nation bloc as long as they have been vaccinated with one of the four shots authorised by the European Medicines Agency — AstraZeneca, Pfizer, Moderna and Johnson & Johnson.
The AstraZeneca shot made in India is not included, nor are other vaccines used in developing countries, including those manufactured in China and Russia.
Individual EU countries are free to apply their own rules for travellers from inside and outside the bloc, and their rules vary widely, creating further confusion for tourists.
So if a person has received a vaccine not approved by the EU and wants to travel to Europe, what can he or she do? Some are, therefore, using booster shots of approved vaccines as a way to get into the continent.
Dr Mesfin Teklu Tessema, director of health for the International Rescue Committee, said countries that have declined to recognise vaccines cleared by WHO are acting against scientific evidence.
“Vaccines that have met WHO’s threshold should be accepted. Otherwise it looks like there’s an element of racism here,” he told AP.
The search for a better vaccine
Before vaccines are introduced in the market, they undergo testing, and the results are released to the public. Despite this, some people feel that they get better protection from certain vaccines. If those vaccines are not immediately available in their country, they are inoculated with whatever is available. Later, when the vaccine of choice is available, some people opt for them as booster shots.
In some countries where vaccines are in short supply, the health authority authorises the mixing of vaccines to protect the people.
Canada’s National Advisory Committee on Immunisations, the body that advises federal health officials on vaccines, has said that the Pfizer and Moderna mRNA vaccines against COVID-19 can be used interchangeably when a second dose of the same vaccine is not readily available.
Why have some countries approved vaccine-mixing?
Several countries, including Canada, Spain, Germany, France, Finland and South Korea, have already approved dose-mixing mainly due to concerns about rare and potentially fatal blood clots linked to the AstraZeneca vaccine.
Vietnam and South Korea too approved the mixing of Pfizer with AstraZeneca vaccines, but that’s more due to the lack of adequate supplies of vaccines.
However, the US Centres for Disease Control and Prevention has warned against mixing vaccines except in pressing situations caused by production or supply problems. In the UK, Public Health England too has taken a similar stance.
Dr Mary Ramsay, the head of Immunisations at PHE, said: “We do not recommend mixing the COVID-19 vaccines — if your first dose is the Pfizer vaccine, you should not be given the AstraZeneca vaccine for your second dose and vice versa.
“There may be extremely rare occasions where the same vaccine is not available, or where it is not known what vaccine the patient received,” Dr Ramsay told the Independent. “Every effort should be made to give them the same vaccine, but where this is not possible it is better to give a second dose of another vaccine than not at all,” she added.
from World,Europe,Asia,India,Pakistan,Philipines,Oceania,Americas,Africa Feed https://ift.tt/3z1RXHL
No comments:
Post a Comment